Polyaniline Dispersion
“Dispersion” is usually underestimated even by scientists. In contrast to wide belief, “dispersion” is not just “mixing” of 2 or more components, but usually requires special machinery to provide enough shear for the dispersion to succeed, and involves quite complex steps on the nanoscale, these steps are taking place more or less at the same time:
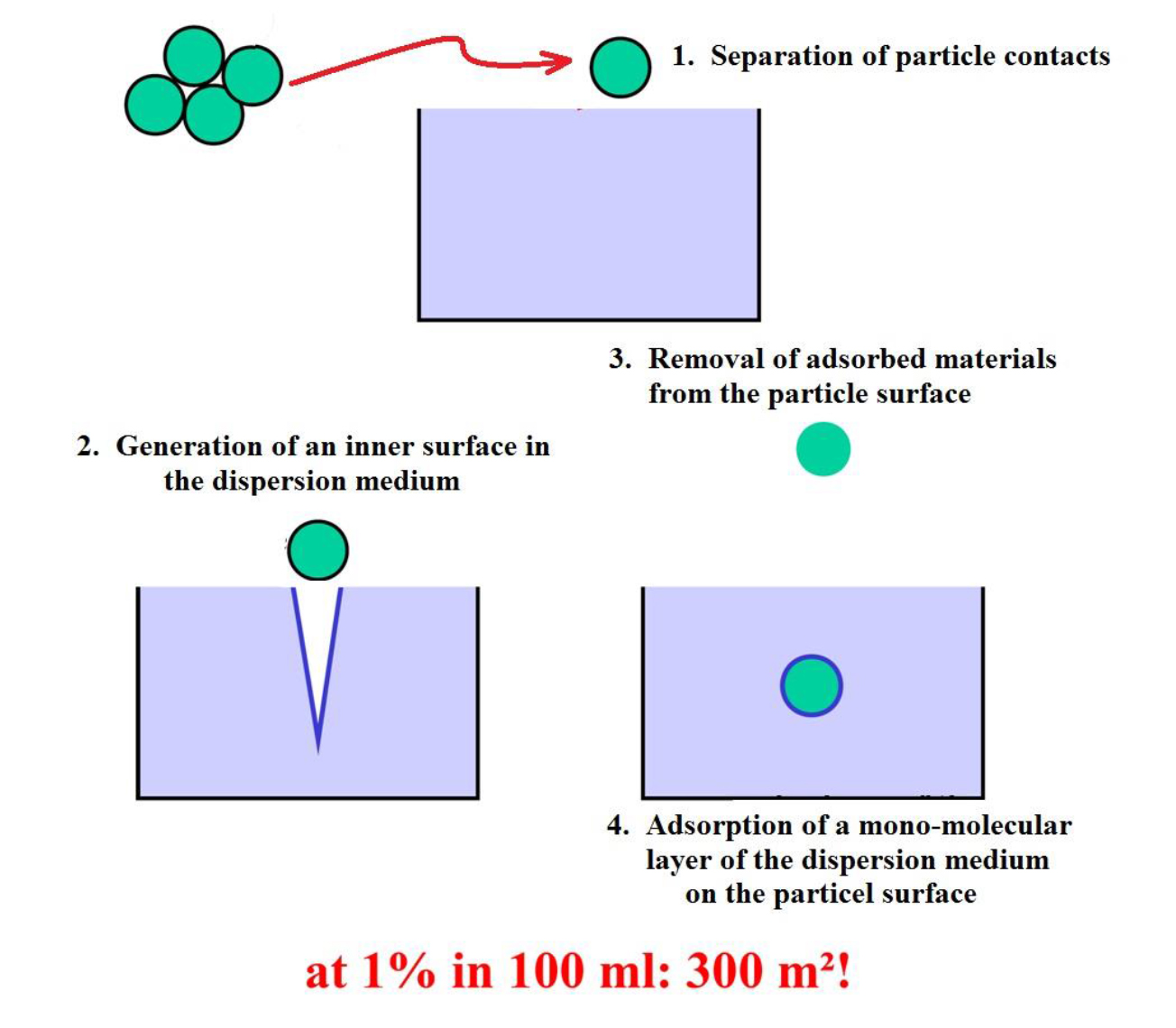
Step 1: The material to be dispersed is available in form of agglomerates. These consist of secondary and primary particles. In case of Polyaniline, the primary particle size is 10 nm only, these can not be dispersed as such in polymeric environment, only in special organic solvents. In a polymer blend, we can achieve around 70 nm (secondary) particle size. These still consist of 10 nm primary particles. First step is the separation of these 70 nm particles from the big agglomerates.
Step 2: In the dispersion medium, internal inner surfaces need to be created. This is done by the turbulent conditions during the dispersion process. Dispersion can not take place in media under laminar flow conditions, turbulent conditions. These inner surfaces will become the “interfaces” after dispersion.
Step 3: At the same time, at least parts of what is adsorbed on the particles’ surfaces will be removed by the dispersion medium. This is at least air and water, partially also all kinds of impurities.
Step 4: That’s the final key dispersion step. The freshly created inner surfaces of the dispersion medium adsorb on the freshly cleaned particle surface. As the result, a monomolecular layer of the dispersion medium will be firmly adsorbed.
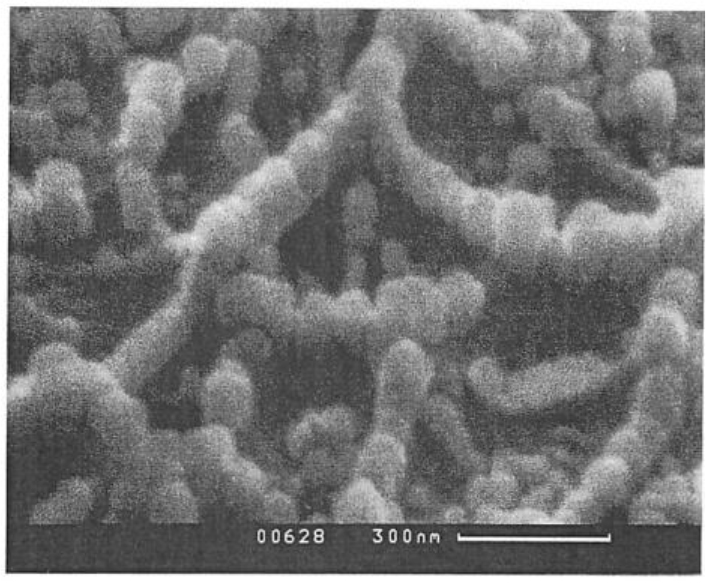
After completed dispersion, and if the dispersed conductive particles are exceeding a certain critical concentration, the formerly isolated particles will form pearl-chain like structures (see SEM photo below) in which the conductive particles are directly contacting so they can conduct electricity. Such structures are self-organizing and are called “dissipative structures”. These structures can be well understood as “non-equilibrium systems”. Bernhard Wessling had created a special non-equilibrium thermodynamical theory for dispersions and emulsions based on the nobel prize winner Prof. Ilya Prigogine’s general non-equilibrium thermodynamics.
For a deeper understanding, we recommend to study the relevant papers in this web site. We are happy to guide you through the various publications if you like, and then please let us know your questions.